PX282 - B2 - typical spectral lines
-
typical spectral lines include
, , ionized metals, and metals - in astronomy, metals are everything but
or
- in astronomy, metals are everything but
-
and metals occupy most of the spectrum -
and ionized metals occupy the hot end, ie: shorter wavelength -
when stars are too hot:
is ionized, therefore there are no absorption lines from it (no electron) has very tightly bound electron, which needs high temperature to raise from the ground state, which allows absorption at visible wavelengths - metals get ionized at high temperatures, and are very prevalent for sun-like stars
-
when stars are too cold:
has a few electrons in state - balmer lines, which are in the visible range, need electrons in
state to be visible - lyman lines, which are formed, are in UV
- molecules are seen only in the coolest stars, as hotter stars break them up
-
key points:
- temperature controls the lines seen, through excitation and ionization
- if lines are not seen, it does not mean that an element is not present
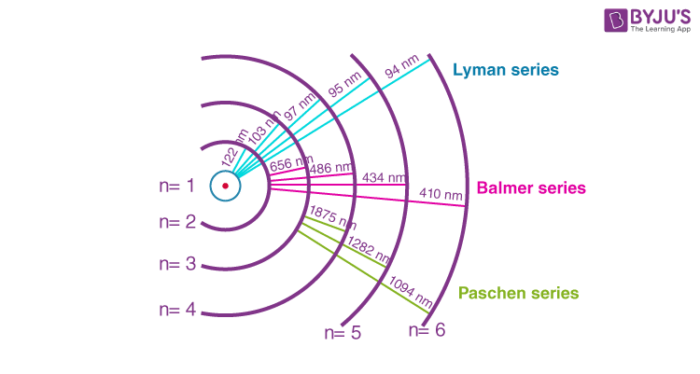
image: byju's