PX262 - H2 - quantum mechanics and many particles
- in classical mechanics, for several particles
, the kinetic and the potential energies are given by:
- in quantum mechanics, the system is defined by a wavefunction, which is a function of the positions and spins of the particles:
- it must satisfy the schrödinger equation:
is the hamiltonian operator:
where,
-
for identical particles,
is either symmetric or antisymmetric -
it is symmetric when the coordinates of any two particles are swapped if they are bosons (eg:
-mesons, phonons, photons, etc)
- and it is antisymmetric when the particles are fermions (eg: electrons, protons, etc)
-
this captures the pauli exclusion principle: no two electrons can occupy the same state
-
eg: putting several non-interacting electrons in a box (1D)
-
the single electron is bound by:
-
the energy is given by:
- the solutions are in the form:
- trapping the electron in the box:
, - therefore, the wavenumbers:
- and the energies:
- the states:
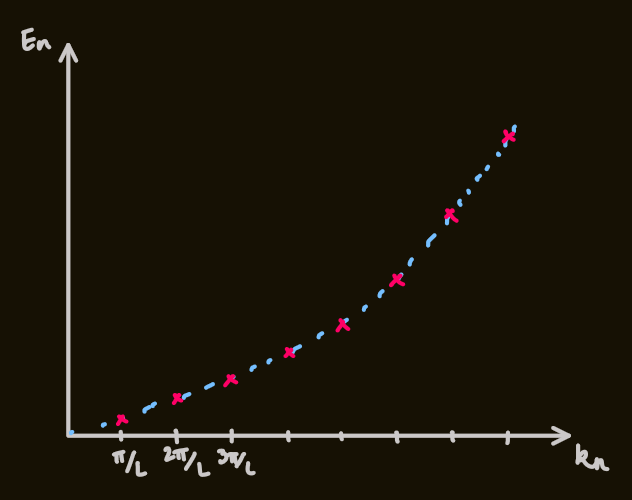
- the ground state energy,
, for a system of several electrons, using the pauli exclusion principle:
where, the two