PX262 - F5 - particle in a spherical potential well
- considering that the particle is bound to be within a sphere, the potential energy would be:
- the radial schrödinger equation:
- solving it for this potential:
- setting a constant:
, and transforming: - this is well known in mathematics as the spherical bessel equation and has solutions in the form of spherical bessel functions,
, and spherical neumann functions,
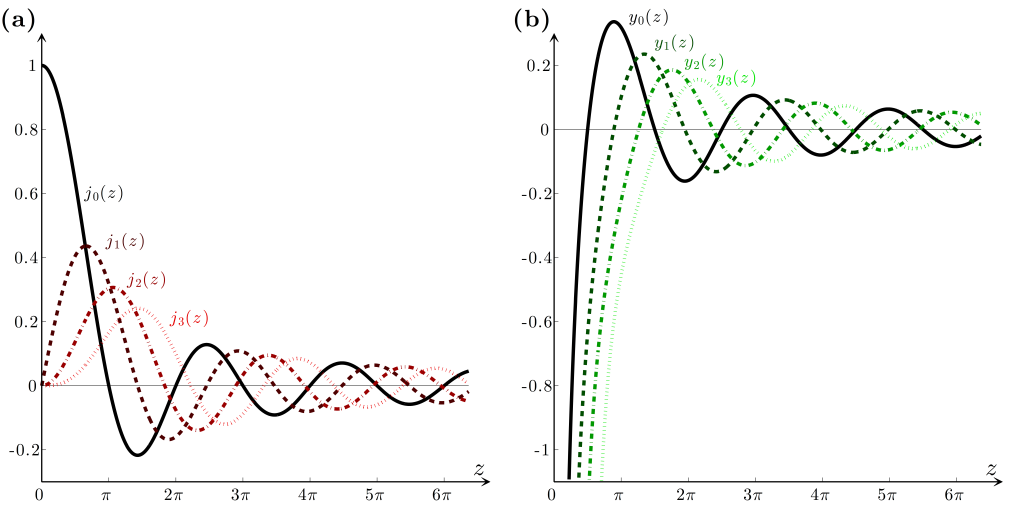
imgae: P. H. Jones, O. M. Maragò & G. Volpe
spherical bessel functions (left), and spherical neumann functions (right)
-
spherical neumann functions diverge at
, and thus, they cannot describe a wavefunction as it cannot be normalized -
therefore, spherical bessel functions are the only valid solutions
-
imposing the boundary condition,
, ie: -
the spherical bessel functions are zero at many points
-
denoting the position of the
crossing of zero by , the energy levels are: -
the wavefunction:
-
there are three quantum numbers,
-
states with different
and/or will have different energies