PX156 - D7 - alpha decay of nuclei
geiger-nuttall law
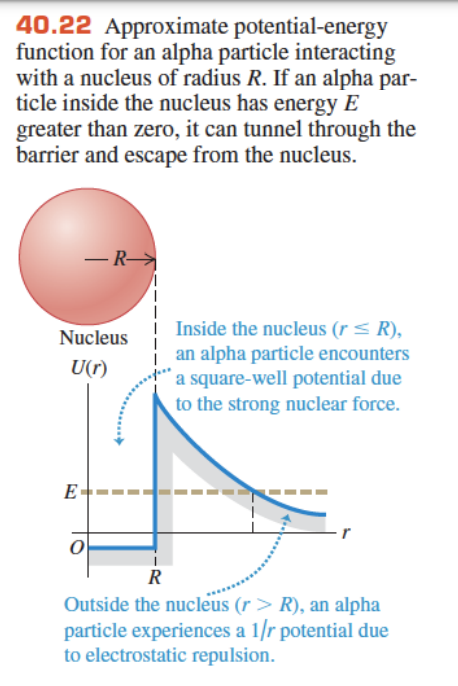
-
the alpha particle is in the potential well of the nucleus
-
a classical particle must have an energy greater than the barrier to escape, but a quantum particle doesn't have to
-
classically, alpha decay cannot be explained
-
alpha decay happens due to quantum tunnelling
-
number of nuclei remaining at time, $$\frac{dN}{dt}= -\lambda N$$
life time
- an
particle: - atomic number of nucleus
- charges
- potential energy:
- if the particle escapes:
where,
- for
where,
- in reality, there are a range of kinetic energies, so to account for that, an integral must be taken:
decay rate = number of attempts at the barrier per unit time
where,
- note:
as well as orders of magnitude, while changes very little ( )