PX156 - A4 - the photoelectric effect
-
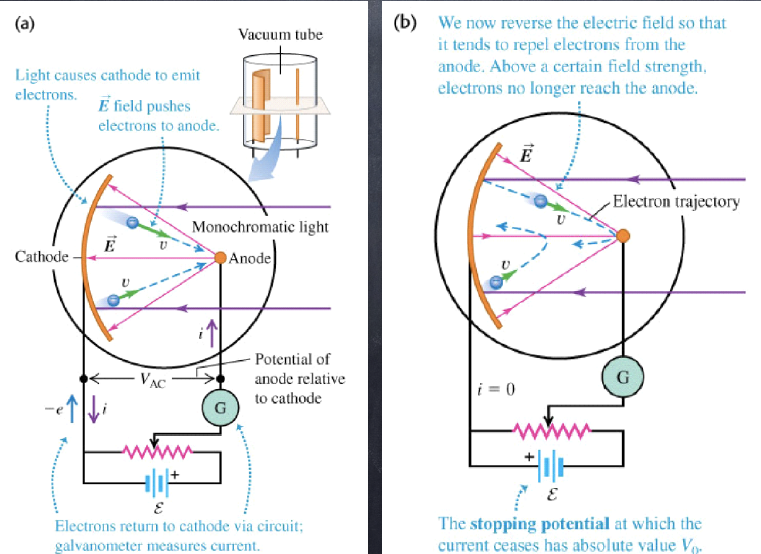
the emission of electrons from a metal surface due to incident electromagnetic radiation
-
lenard's experimental observations:
- not all electrons emerge with the same kinetic energy
- the maximum kinetic energy is independent of the intensity of light, but dependent on the frequency
- no electrons are emitted if the frequency of light is lower than a critical value, the threshold frequency
where,
- for a photon with
,
- considering photon as a particle:
where,
- for
if
- for