PX154 - E6c - adiabatic processes
reversible
- however,
for an adiabatic process, so:
- isentropic process: entropy doesn't change
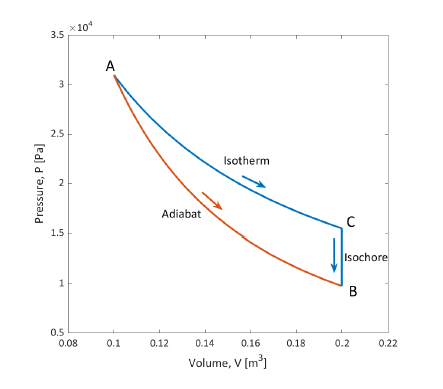
irreversible
- free expansion of an ideal gas [YF ex 20.8]
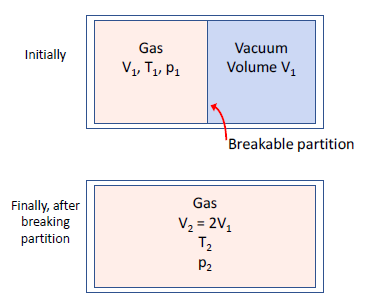
- process is adiabatic:
- no work is done by the gas as there is no piston to push:
- no change in internal energy:
- does the entropy change?
- process is irreversible, so, come up with a reversible isothermal process that achieves the same change in state
- start and end points identical, but, the isothermal process requires:
- the entropy increases