PX154 - E6a - introduction to entropy
[YF 20.7]
- eg: from here:
is irreversible - the system becomes more random:
- initially sorted into hot and cold regions
- energy "mixes", meaning it is more disordered/random
- the system has gained entropy
- eg: a reversible isotherm
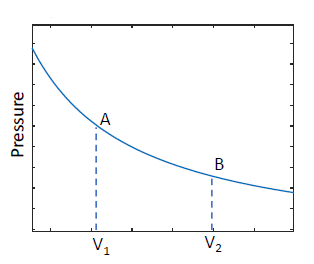
- from
is added to do work - at
molecules are able to move around in a larger volume, so, system is more disordered higher entropy - returning to
, gas is exactly the same as it was initially, so, for is a function of state doesn't depend on the path taken
- from