PX154 - E5 - reversible and irreversible processes
heat transfers
- consider a heat engine placed next to a cold engine
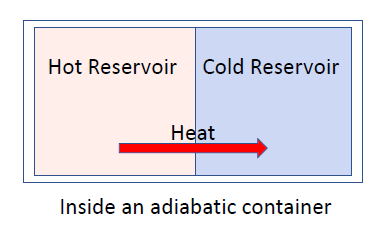
- initially, the objects are not in equilibrium and heat is transferred from hot
cold until they have the same temperature
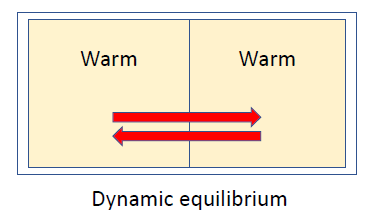
- heat will not be transferred to return to the first state of hot and cold, so, the process is irreversible
isothermal and adiabatic processes
-
in the study of the carnot cycle, an isothermal expansion was first considered
-
if the reservoir is the same temperature as the engine (
), then there is no heat transfer, so, , and nothing happens! ( ) -
set
in order to obtain , but this is no longer reversible since heat is transferred from warmer reservoir to the cooler engine -
for an isothermal process an infinitesimally small difference in temperature is required so that the process could be reversed by an infinitesimally small change in conditions
-
process has to be slow so that the engine can always be considered to be in equilibrium
-
why does this matter?
- if
then , and some of will go to work done, however, some of the heat, , will warm the engine - so, the work done,
, is reduced, which reduces efficiency - therefore, the reversible isotherm gives the maximum
for a given
- if
-
adiabatic cooling (step
in the carnot cycle) - still an expansion: engine doing work
- cooling from
is not known at any given time, but heat transfer to reservoir cannot be allowed to occur as this would be irreversible - if
, then again is maximized - if
, energy is lost to the reservoir reducing efficiency
conclusion
- the most efficient cycle is reversible and consists of isotherms and adiabats
- however, all real processes are irreversible and proceed in a particular direction
- the first law doesn't tell this
- the second law of thermodynamics does