PX154 - D4e - adiabat
adiabatic expansion of an ideal gas
- rearrange:
- note: for
- integrate:
$$TV^{\gamma-1}=constant$$
- using ideal gas law:
$$pV^{\gamma}=constant$$
- compare with the isotherm:
- here,
with constant - so, for isotherm:
- here,
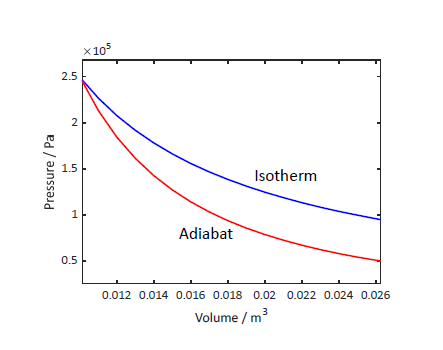
work done along the adiabat
- we have,
, since - take
, here, ( )
$$\therefore W = -n C_{V} \Delta T$$- can also be written as:
- note:
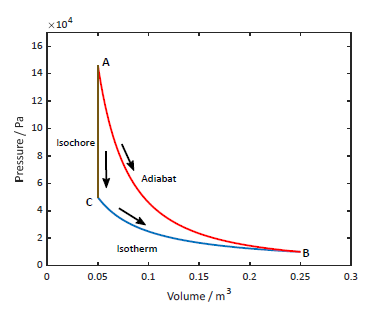
- whether we go
via adiabat or , is the same is the same - for
: - isotherm:
- isochore:
- isotherm:
- for adiabat:
heat capacity at constant volume, even though, V is not constant