PX154 - D1 - introduction
some concepts
- ideal gases are considered
- systems:
- consider a specific system in a closed container
- no particles enter/leave
is constant
- no particles enter/leave
- volume,
, must be changeable
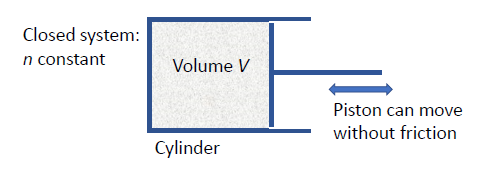
- a piston that moves without friction and with a perfect seal is used
- diathermal wall: perfect heat transmission through wall via conduction
- adiabatic wall: perfectly insulating, no heat transmission, often depicted as double wall
- states:
- the state of a system is given by the state variables at any given time
- intensive variable - extensive variable - intensive variable - extensive variable (entropy) - extensive variable
- intensive and extensive correspond to whether the variable changes with a change in the size of the system. so, if a system has twice the volume, it has twice the mass, but the temperature is the same.
- the state of a system is given by the state variables at any given time
- consider a specific system in a closed container