PX154 - C9 - heat capacity of the ideal gas
[YF 18.6]
relating heat capacity
- need to relate the heat capacity of our gas to its motion (the motion of its molecules)
- two definitions to consider:
heat capacity at constant volume heat capacity at constant pressure
heat capacity at constant volume
- for now, consider
:
- if some heat,
, is added, the kinetic energy of the gas increases:
- from here:
- for
molecules:
- and for
moles:
used instead of - kinetic energy owing to translation
- at
, measured values:
| gas | |
|---|---|
| He | 12.5 |
| Ar | 12.5 |
| Ne | 12.7 |
| Kr | 12.3 |
| 20.8 | |
| 20.4 | |
| 21.1 |
- key observations:
- noble gases - monatomic
- other - diatomic
- noble gases - monatomic
- reason:
- monatomic particles only translation
- for diatomic, there may be rotation and vibration
equipartition of energy
- each form of motion is a "degree of freedom" and contributes to
of energy per atom
translation
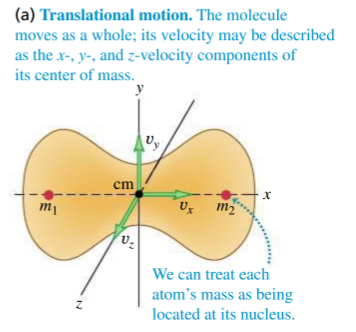
- our translation is in 3D, and translation in each of these is a degree of freedom contributing
- translation in 3D contributes to a total energy
per atom - for
moles, :
rotation
- neglect this for monatomic gases
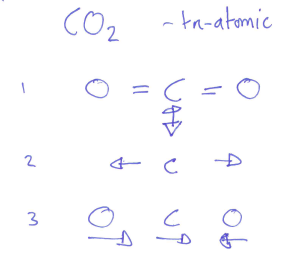
- diatomic gases:
- molecule can rotate, but not about all 3 axes:
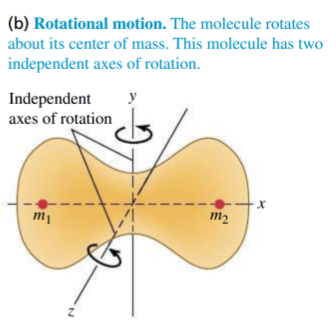
- it is found that rotation can only be observed about
and axes - one of these axes is along the molecule's axis, effectively the same as the monatomic case, so there is no contribution
- this gives 2 degrees of freedom
per atom per mole
- for
, an extra is obtained
- molecule can rotate, but not about all 3 axes:
vibration
-
doesn't exist for monatomic molecules
-
molecules can vibrate via stretching/contracting, or sometimes by bending of the interatomic bonds
-
the bond can be modelled as a spring and treat it as a harmonic oscillator, SHM
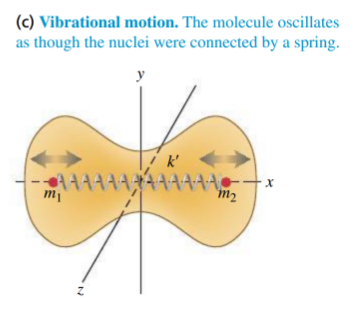
-
and from the vibration have to be included. each adds one degree of freedom - total
- contribution to
is
- total
-
at room temperature, vibration doesn't contribute to the heat capacity
-
vibration is quantized
- requires a quantum mechanical model PX262 - 0 - quantum mechanics and its applications
- eg: for
, minimum energy to be absorbed to increase vibration is - using
, this corresponds to a temperature of
-
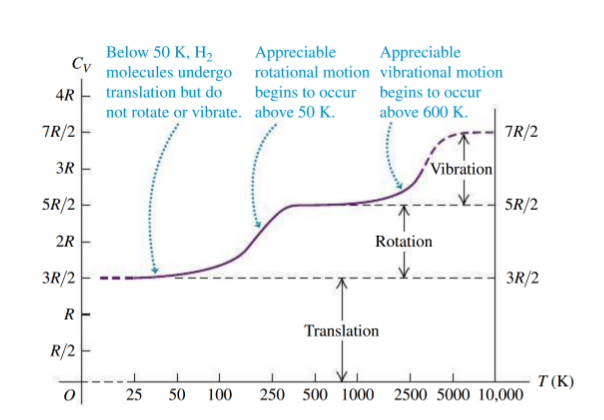
-
comment:
- for larger molecules, vibrations are more complicated with different modes available
at