PX154 - C7 - maxwell-boltzmann distribution
molecular speeds and energy
- the molecules in a gas have different speeds, and we define a distribution function [YF eqn 18.32]
-
for
at three temperatures:
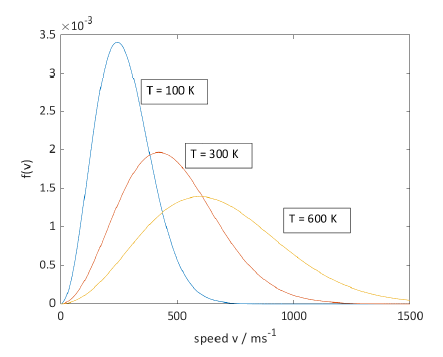
-
as
increases: - peak of the distribution moves to higher speeds
- the distribution also becomes broader
- some slow molecules at high
, conversely some fast molecules at a low
-
if we have
molecules, the number whose speeds between and is: [YF p627 ]
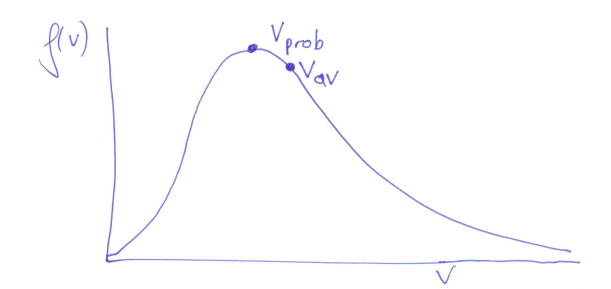
-
is the most probable speed -
is the average speed = -
- fits with our kinetic model for the ideal gas and its prediction for
at for
- fits with our kinetic model for the ideal gas and its prediction for
-
we can find the energy distribution too:
-
using , we get:
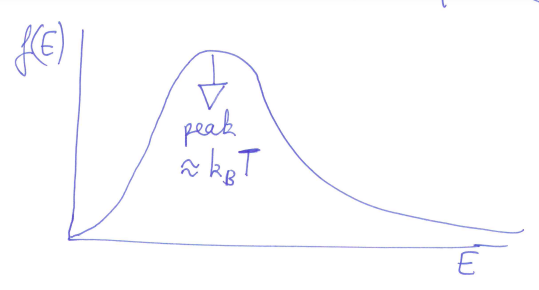
-
gives the energy associated with a given temperature - at
- at