PX154 - C6 - kinetic-molecular model of the ideal gas
- links the ideal gas equation to the motion of the gas molecules
derivation
- how collisions of the molecule lead to pressure
- consider a volume of
filled with an ideal gas of molecules, each of mass
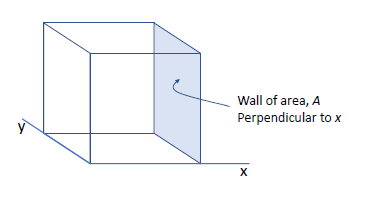
- firstly, consider just one molecule of gas moving in 3D with a velocity of
- consider an elastic collision with the shaded wall
- in the collision,
component is reversed but and components are unchanged - the change in momentum is then
- we need to know the rate at which collisions occur with the wall for the gas
- how many molecules hit the wall in a time
? - molecules have an x-component of velocity
- in time
, they can move a direction of , so, only molecules less than can hit the wall
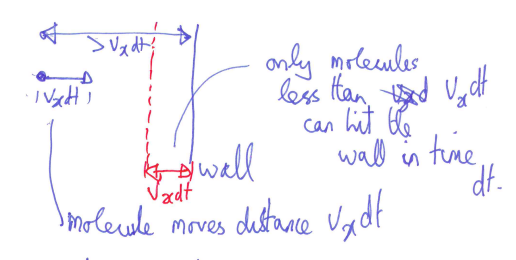
- where,
- only half the molecules are likely to be moving towards the wall at given time
- so, change in momentum
in time :
- this causes a force [YF eqn 18.10] :
- so, the pressure exerted on the wall is:
- we apply the same argument for
and - in an isotropic system,
, ie: :
- we have assumed that all
molecules have the same speed , however, we will see that the molecules have a distribution of speeds here - here, we are using
, so, we will use the average value of
- multiplying both sides by
:
alternative method
PX155 - A2 - newton's second law:
- if length of the cube is
, time for a molecule to get from one wall to the other and back:
- for
molecules:
- statistically,
some consequences
[YF p619]
- we can define temperature:
- microscopic definition of temperature
- we can consider kinetic energy:
- we have related the average KE of the gas to its temperature