PX154 - C5 - phase diagrams
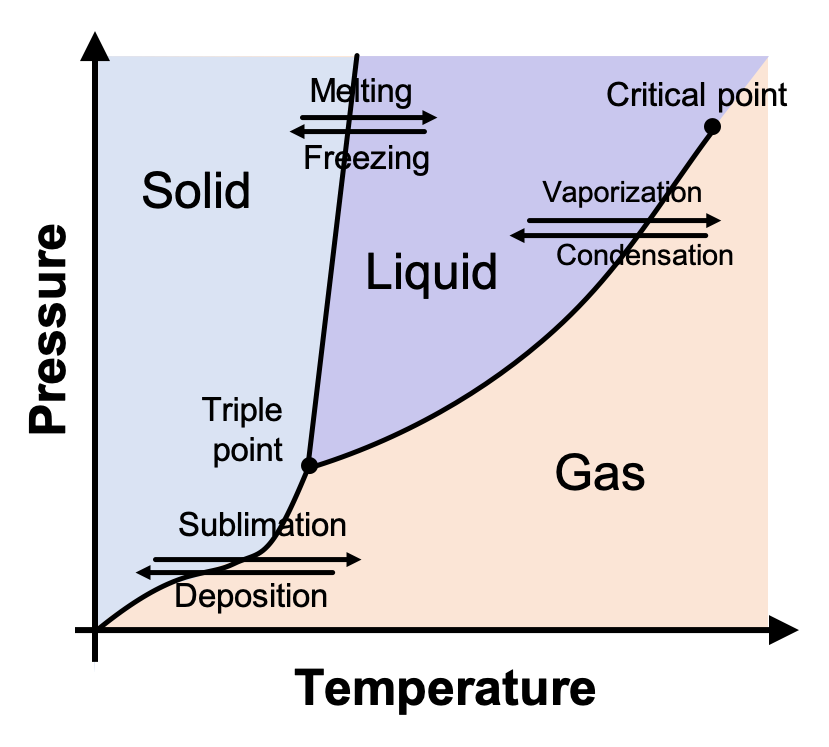
- 3 phases separated by phase boundaries
- at the triple point, where the phase boundaries meet, all three phases coexist
- there is no liquid phase below the pressure of the triple point, and solid sublimes into the gas
- eg:
"dry ice":
- eg:
- there is always a phase boundary between solid and liquid at the highest pressure studied
- gas liquid phase boundary ends at the critical point
- no distinction between gas and liquid at temperatures/pressures above this